Research
The overarching goal of my research program is to understand the role of immune cells, senescent cells, and matrix mechanics in tissue regeneration and regulate them by engineering immunomodulatory materials and drug delivery vehicles to restore musculoskeletal tissues impaired by diseases, trauma, and aging. To this end, we:
1) Elucidate immune dysregulation and identify predictive biomarkers for musculoskeletal disease and trauma
2) Develop hybrid nanovesicles for targeted delivery of disease-modifying drugs
3) Study the influence of soft matrix mechanics on immune cell phenotype and function
4) Study the mechanobiology of macrophages and modulate their phenotype using biophysical cues
We use high-throughput modalities, such as single-cell and spatial transcriptomic modalities, imaging methods, \textit{in vitro} and \textit{in vivo} models, and computational and bioinformatic tools to achieve our aims. I have developed productive collaborations with engineers, clinicians, biologists, and bioinformaticians for carrying out my multidisciplinary work.
Below, I summarize my primary research projects and future goals.
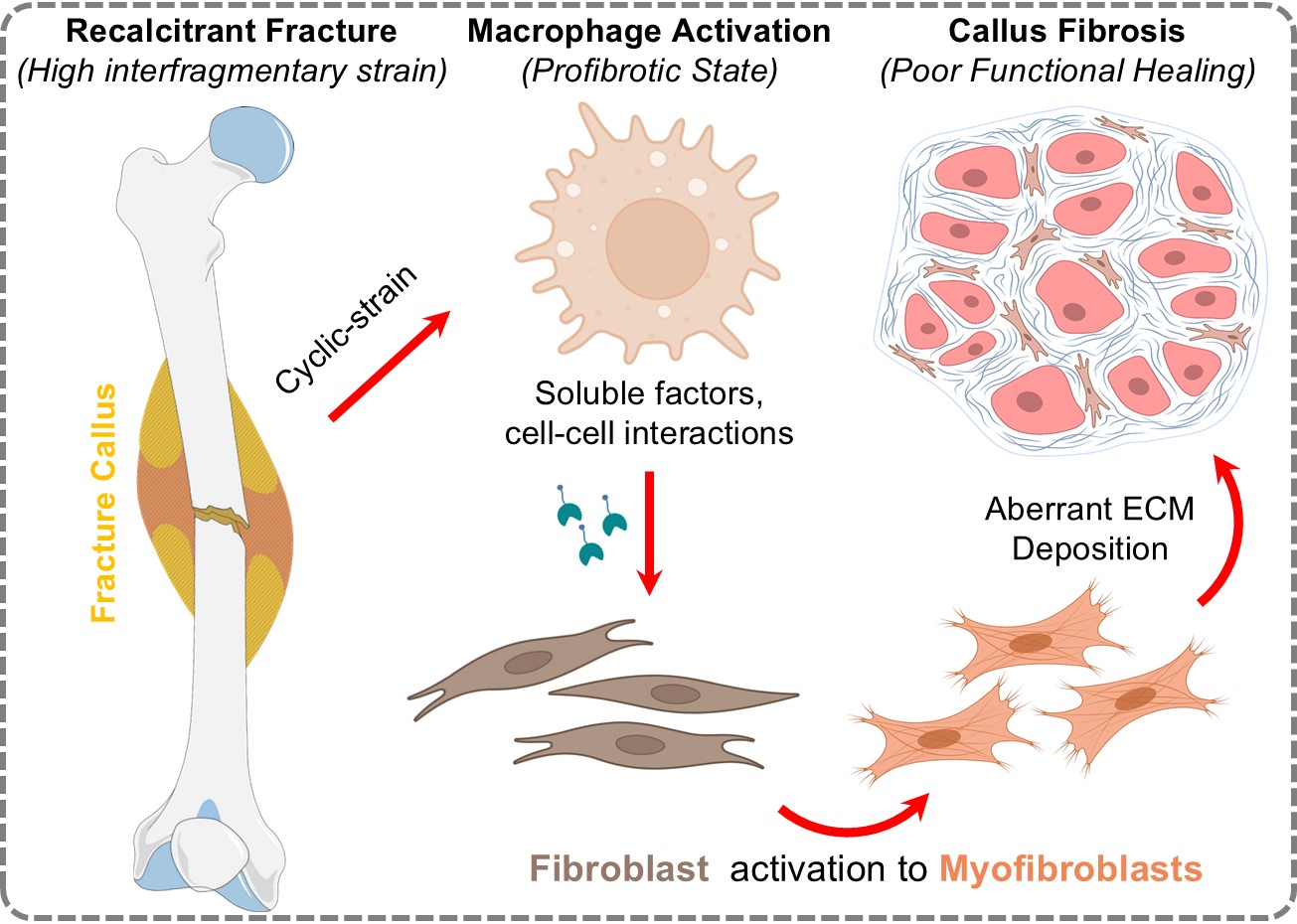
i) Targeting Macrophage Driven Fibrosis in Bone Repair and Healing
Fracture nonunion impacts up to 10% of long bone fractures in the U.S., causing chronic pain and disability. Current treatments focus on bone stabilization but overlook pathological fibrosis—the main biological barrier to healing. Our research identifies macrophage derived thrombospondins and galectins as key drivers of fibroblast activation and scar formation, particularly in unstable fractures. We propose that high mechanical strain prompts macrophages to release these profibrotic factors, leading to fibrotic nonunion. Using advanced fracture models, mechanobiology, and omics, our team is working to disrupt this immune-stromal cross-talk and enable functional bone regeneration without fibrosis.
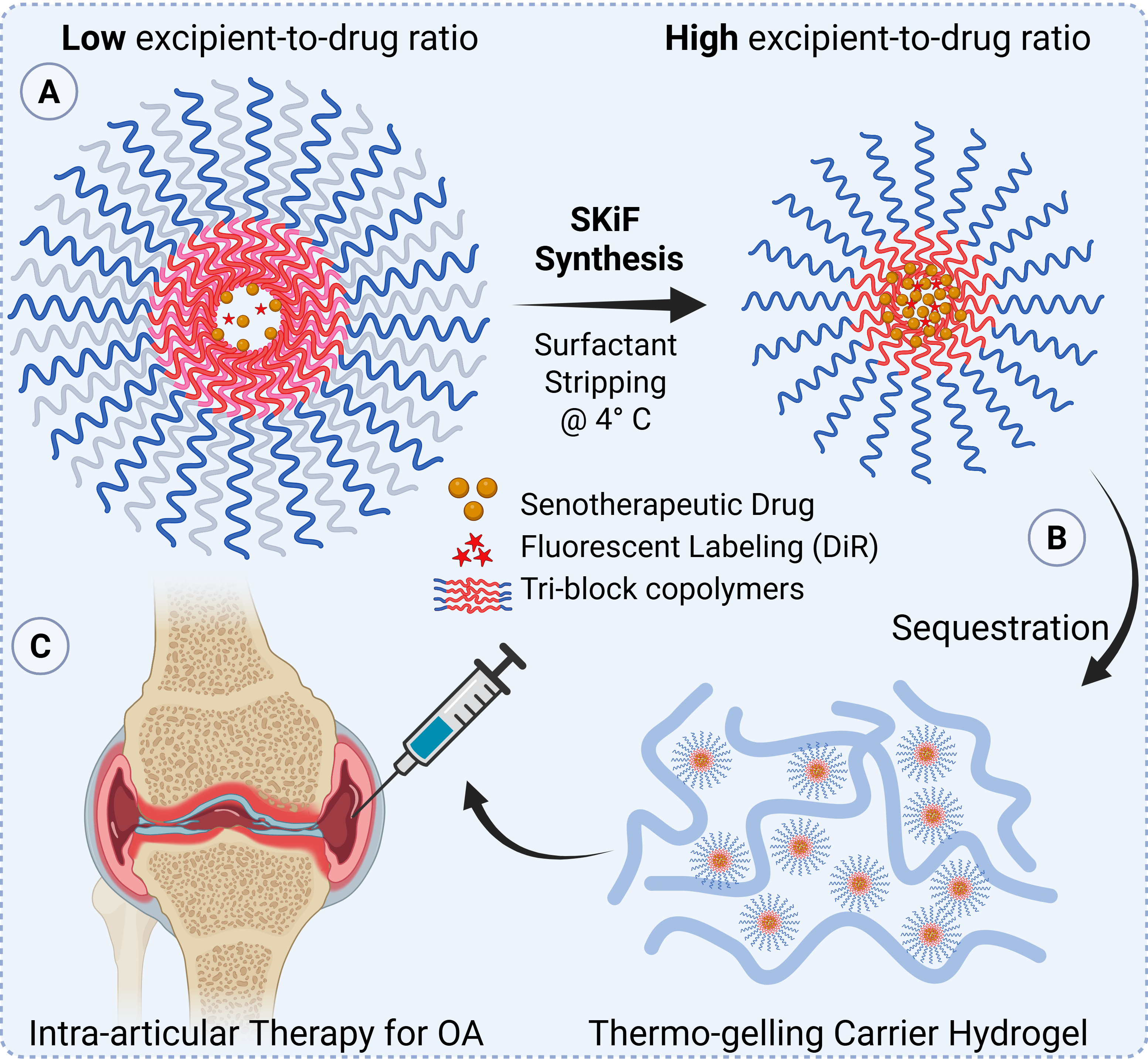
ii) Targeting Cellular Senescence in Osteoarthritis
Osteoarthritis (OA) and post-traumatic OA (PTOA) cause significant disability, yet current treatments only manage symptoms, failing to address the root causes of cartilage degeneration. Our research focuses on chondrocyte senescence—a state where cartilage cells secrete harmful factors, disrupting joint health. We are developing a novel drug delivery system that targets a key cellular pathway known to suppress this senescence. By encapsulating a therapeutic molecule within advanced nanocarriers and embedding them in a biocompatible hydrogel, we aim to achieve potent, sustained delivery to the joint, promoting cartilage repair and offering a disease-modifying approach for OA.
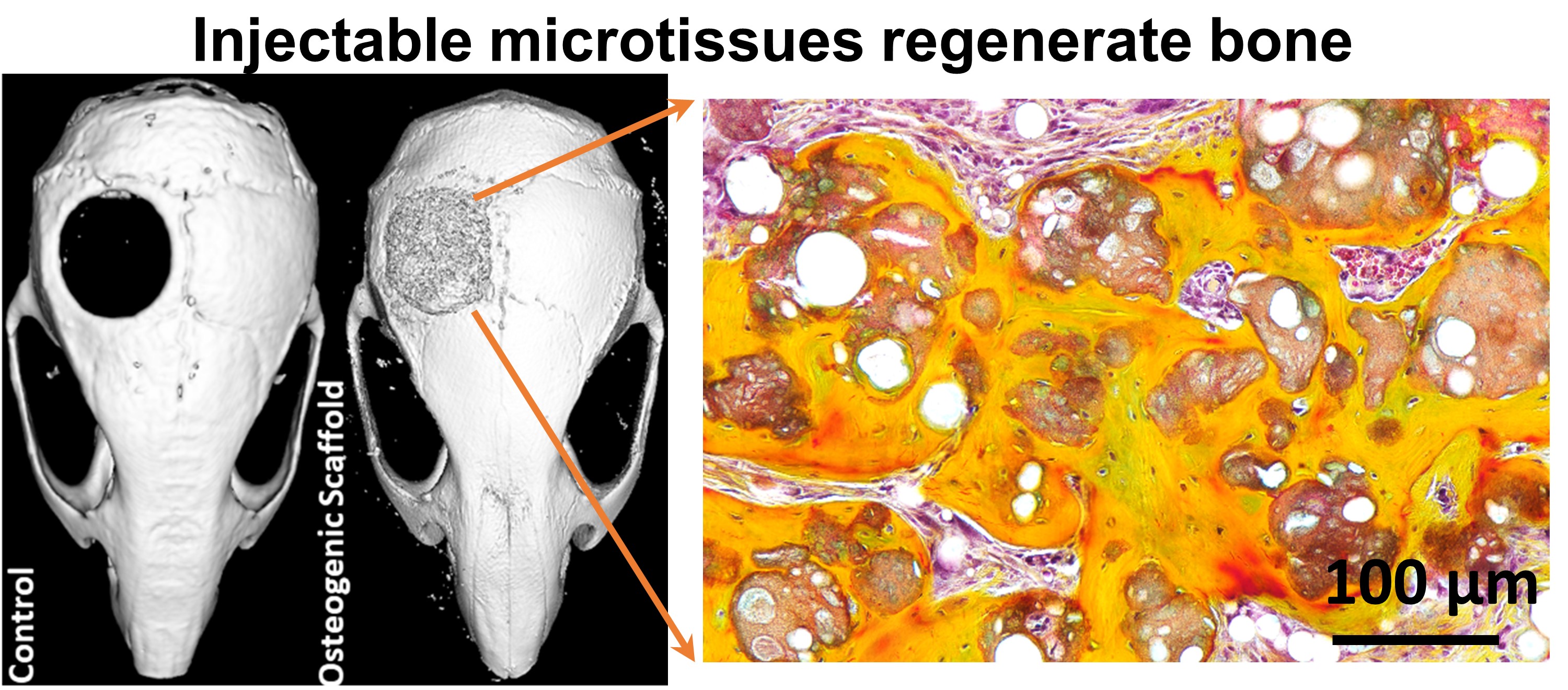
iii) Injectable Therapies for Bone Defects
Nonunion fractures are difficult to treat and often lack effective solutions. Our lab is advancing injectable stem cell therapies for bone regeneration, moving from small animal models toward human trials. To improve clinical outcomes, we are studying how immune cells like macrophages guide bone healing. By harnessing the body’s own inflammatory signals, we aim to design injectable therapies and scaffolds that promote bone repair without relying on external growth factors, bridging the gap between immunology and regenerative medicine.
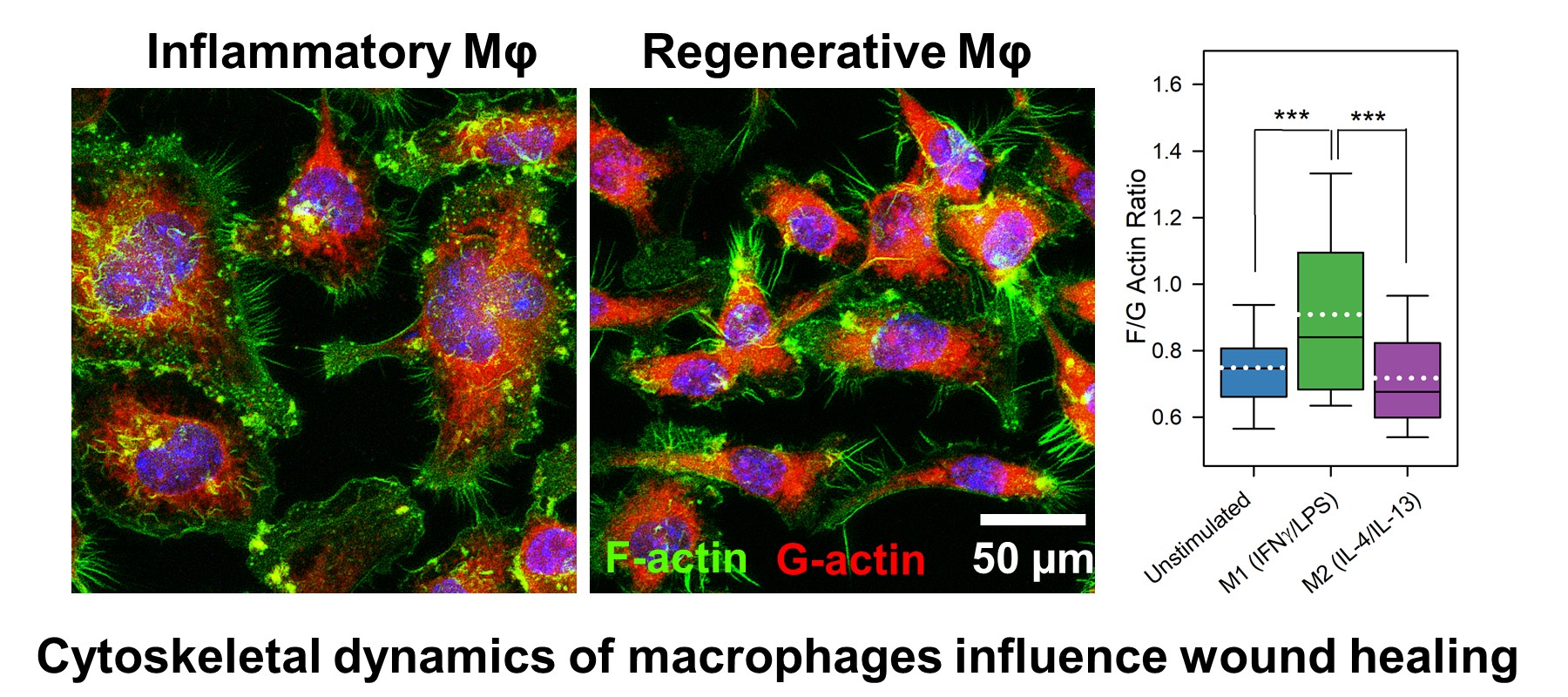
iv) Biomaterials for immunomodulation and immunoengineering
After injury or infection, immune cells like macrophages and neutrophils rapidly infiltrate tissues, guided by their secretion of MMPs. Our research develops “smart” biomaterials that sense and respond to inflammation, enabling precise, on-demand release of drugs or growth factors. This targeted approach prevents drug washout and prolongs therapeutic effects, especially for chronic conditions like osteoarthritis. We are also interested in modulating immune responses by tuning material properties such as curvature, stiffness, viscoelasticity, and porosity, allowing us to further direct healing and tissue regeneration.
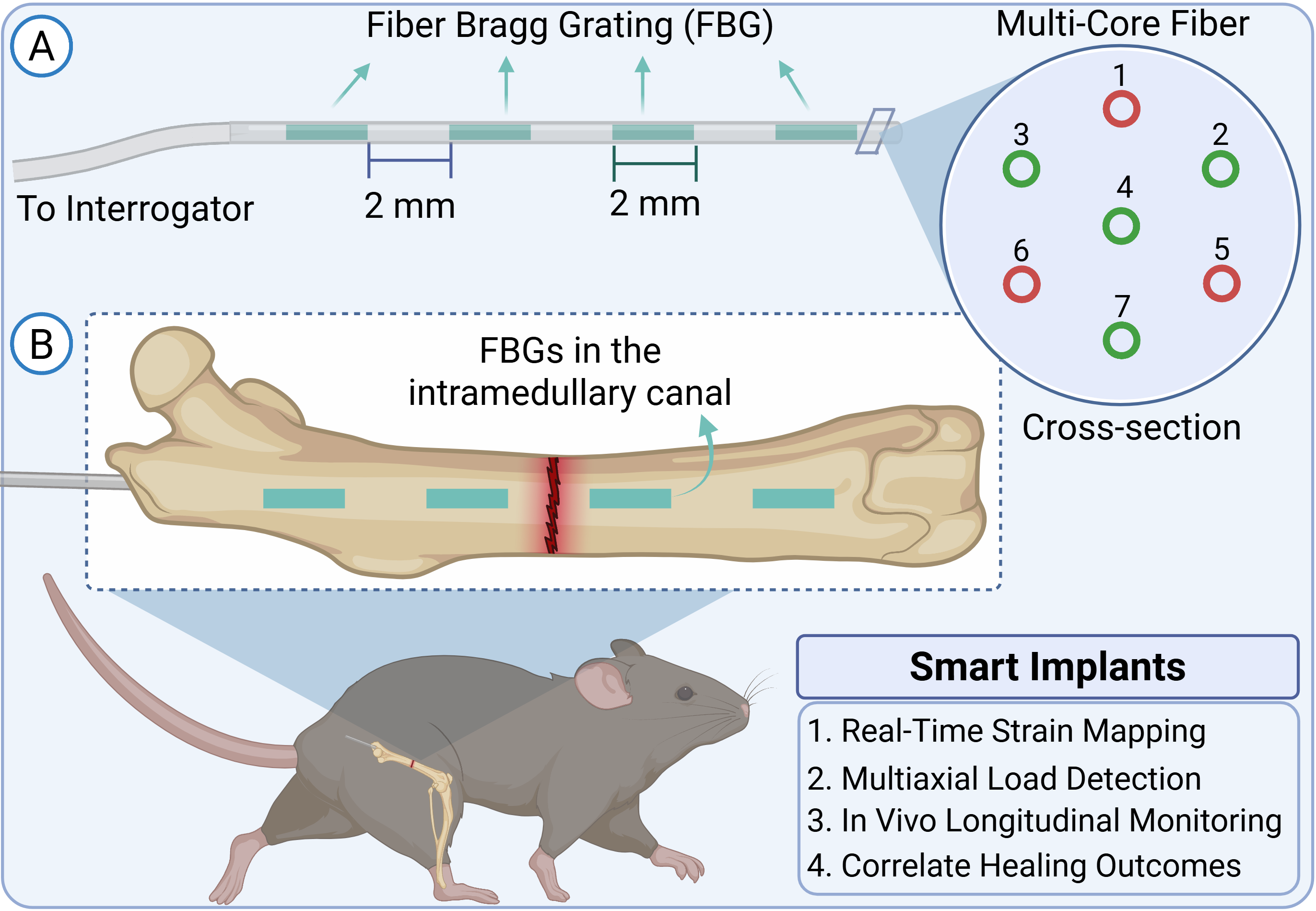
v) Real-Time Sensing of Bone Healing with Implantable Optical Strain Sensors
Orthopedic implants are vital for repairing long-bone fractures, but clinicians and researchers currently lack tools to monitor the mechanical environment inside healing bone. Excessive strain at the fracture site can disrupt healing and lead to poor outcomes, yet standard imaging methods only provide static images and miss dynamic changes. Our team is developing a miniaturized, implantable optical strain sensor using advanced fiber Bragg grating technology, integrated into a nitinol intramedullary nail for use in small animal models. This device will enable real-time, 3D monitoring of mechanical strain during bone healing, allowing us to directly link biomechanics with biological responses. Our goal is to create a scalable system for studying how mechanical forces influence tissue regeneration and fibrosis, ultimately guiding the development of better therapies for bone repair.